For all practical purposes, high-level radioactive waste will remain radioactive forever. It is common to mention that high-level radioactive waste needs to be stored from thousands to millions of years due to the long half-live of radioisotopes in the waste. But why? To understand what high-level radioactive waste is, first it is necessary to review some concepts of radioprotection.
What radioactivity is
Radioactivity is the random and spontaneous decay of unstable atoms. This means that an atom changes to another one and in the process emits alpha and beta particles, and gamma-rays. The radioactivity is easy to measure by counting the number of atoms that are decaying in time and this is called the activity of the radioactive material. If one decay occurs per second, then the radioactivity of the sample is 1 Bq (Bequerel).
A banana has an activity of 18 Bq meaning that 18 atoms of potassium-40 are decaying per second. Carrots and potatoes have a specific activity of about 130 Bq/kg, our own body has an activity of 40 to 70 Bq/kg, Plutonium-239 has 2 300 000 Bq/kg and uranium-238 has 12 400 000 Bq/kg.
Taking only the activity as a metric can lead to serious misinterpretations. For example, it is not the same to be struck by one bowling ball per second falling one meter, as to be struck by 1 000 000 of salt grains (equivalent to one cup) per second falling one meter.
The meaning of half-life time
The half-life time means the time that takes the initial number of radioisotopes to decay to the half of the initial number of atoms. For example, a banana contains about 49 micrograms or 7×1017 atoms of potassium-40 with a half-life of 1 248 000 000 years. This implies that about 18 atoms per seconds at decaying in the banana that we are eating every day.
The activity of a radioisotope decays quite fast with time, in ten times the half-life about 0.01 % of the initial atoms will remain. Then, if we wait 10 times 1.2 x 109 years for eating the banana, then about 1 atom per minute will decay, and if we wait 20 times 1.2 x 109 years, then about 1 atom per year will decay. Formally, the banana will continue being radioactive until the last atom of potassium-40 despairs. Plutonium 239 has a half-live time of 24 110 years, and it is commonly mentioned in discussions related to the need of thousands of years for the storage of high-level radioactive waste.
However, taking only the half-life time as a metric can lead to serious misconceptions. It is not the same to be struck by one bowling ball per second falling one meter for 24 000 years, as to be struck by 1 salt grain per second falling one meter during for 24 000 years.
Radiation shielding and alpha, beta and gamma radiation
The bowling ball or the salt grain mentioned before were illustrating that the radiation is not all the same and it can be divided into alpha and beta particles and gamma-rays. To protect an area/person from a radiation source, for example a banana, a radiation shield is placed as a barrier. Each radiation type has different characteristics.
Alpha particles consist of two protons and two neutrons. Alpha particles will lose their energy in a few centimeters in air and the alpha particles can be stopped by a sheet of paper or by the death layer of cells in our skin. If alpha-emitters are inhaled or swallowed, they can damage sensitive living tissue. However, the damage depends on the activity of source and how the source is assimilated by the body. Every day, we breath, drink and eat alpha emitters for example contained in potatoes and carrots. Plutonium-239, commonly mentioned in discussions related to high-level radioactive waste, is considered a pure alpha-emitter as the accompanied gamma radiation is low intensity. You can hold Plutonium 239 in your hand using a glove or washing your hand after touching it. Alpha particles are not really a radiation hazard unless you eat them.
Beta and positron particles are fast-moving particles with a negative electrical charge (beta) or a positive electrical charge (positron). These particles can lose their energy in about 2 meters, or they can be stopped by a layer of clothing or by a thin layer of aluminum. Some beta particles can penetrate the skin and cause damage as skin burn. If beta emitters are inhaled or swallowed, they can damage sensitive living tissue. The damage will depend on the activity of the source and how the source is assimilated by the body. We eat bananas that contains potassium-40 which is a beta emitter. The process of stopping the beta particles can lead to an electromagnetic radiation called bremsstrahlung. To minimize the bremsstrahlung radiation a shield will consist of a layer of plastic followed by a layer of lead for absorbing the bremsstrahlung radiation.
Gamma rays are packets of energy called photons and are often emitted along with alpha or beta particles during radioactive decay. Gamma radiation have a high penetrating power that a dense material like lead or concrete is required to stop them. Gamma rays can pass completely through the human body and cause ionization damage on the tissue depending on the exposure. Every day, we are exposed to gamma radiation from radionuclides of the uranium-238 and thorium-232 decay series as well as potassium-40 depending on the type of soil and geological formation. The thickness of material necessary to lower a gamma ray to one tenth of the initial level is called tenth-value layer. For shielding cesium-137, the tenth value layer is 2.16 cm using lead while 15 cm in concrete.
So, given a radioactive source, gamma rays are the most harmful external hazard and can pass through a person damaging cells in their path while beta can only partially penetrate the skin causing burns. Alpha and beta particles are most harmful when swallowed, inhaled, or injected. The number of struck depends on the activity of the source. And how long the radioactive source will last depend on the half-life time.
High-level radioactive waste: where does it come from?
When spent nuclear fuel is removed from the reactor the level of gamma rays is too high that can be lethal within minutes of direct exposure. After the fuel assembly is removed from the reactor core, it is placed in a water pool which keep the fuel refrigerated and acts as a radiation shield. After 10 to 20 years in the water pool, the fuel can be moved to a dry cask. The dry casks are designed to lasts 100 years and they can stand up from a direct train impact to a missile impact.
The spent nuclear fuel can be reprocessed by which 96 % of the fuel is recycled and the rest is the high-level radioactive waste. This high-level radioactive waste can be vitrified can then store again in dry casks from where it can be disposed in geological repositories. This technology is mature and used by France, Japan, Russia, UK, and USA. According to Orano La Hague in France, 75 reactors worldwide have used or already use reprocessed uranium. The vitrification can immobilize of high-level radioactive wastes and provide a durable and environmentally stable material.
High-level radioactive waste explained
High-level radioactive waste contains fission products and actinides. Fission products are atoms produced by the fission of atoms. Actinides are elements in the periodic table from actinium upwards. Uranium and plutonium are grouped as major actinides. Then neptunium, americium and curium are grouped as minor actinides. The mass of the remaining actinides (actinium, thorium, protactinium, berkelium, californium, einsteinium and fermium) is too small that are considered as minor trace contaminants. These minor actinides are generated by capturing a neutron transmuting to a heavier atom.
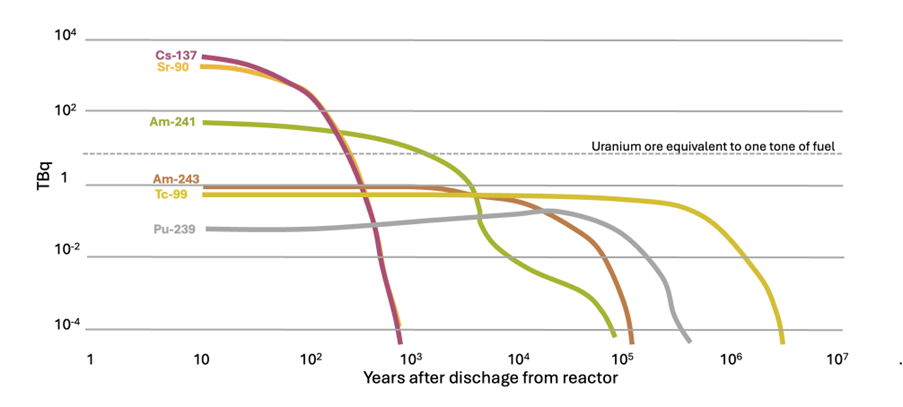
High-level radioactive waste contains fission products decaying with strong gamma radiation and minor actinides decaying mainly with alpha radiation. The figure below shows an example of the radioisotopes present in the high-level radioactive waste in terms of the activity including plutonium and uranium as a reference. Based on the activity, the first two elements are the fission products cessium-137 (gamma emitter) and strontium-90 (beta emitter) both with a half-life of 30 years. These two elements are the most relevant from the radioprotection point of view, but after 300 years the activity will drop to 0.01 %.
Then, next in activity are the actinides americium-241 (half-life of 432 years), americium-243 (half-life of 7 350 years), and plutonium 239 (half-life of 24,000 years) which are alpha emitters. Then, it is followed by the fission product technetium 99 (half-life 213000 years) that is a low-energy beta emitter, and then alpha emitter actinides with longer half-lives such neptunium 237. The rest are radioisotopes with very small contributions.
How dangerous is high-level radioactive waste?
A direct exposure to fresh high level radioactive waste can be lethal within minutes of direct exposure. However, if the high-level radioactive waste is stored in a dry cask, then you can be standing near the casks or even hug the cask. After 300years, most of the strong gamma radiation, mainly coming from cessium-137 and strontium-90, is gone. Then, after 300years if the high-level waste is without any protecting shielding the doses at 1m is of about 4mSv/h so staying one hour at 1 m will be comparable to a CT-scan, but at more than 10m the radiation will be comparable to the natural radiation. Then, after 500 years you can be standing close to the high-level waste, but you should not swallow it as it is harmful.
Small bottom batteries in our home are an example of waste that is around us that are very dangerous if they are swallowed. Only in the US more than 70 children have died, and tens of thousands have required attention in emergency rooms, after swallowing button batteries. Contrary to the bottom batteries in our homes, the heavy atoms in the high-level waste are not very mobile or soluble particularly when they are vitrified, placed inside a container for shielding, and then stored up to 5 km under the surface in a geological repository.

The scale of the generated high-level radioactive waste
Let´s assume that for the next 80 years, a population of 5 million uses only nuclear power for household consumption. Then, in 80 years the total volume of high-level radioactive waste generated (assuming reprocessing) will be a cube of about 3.5m side. The high-level waste is not just compact into one block but conditioned with multiple layers of protection. This volume is quite small when compared to volume produced from other power generation and general industries. This is a direct consequence of the high-power density of the nuclear generation. In the case of coal about 20 % in weight of the fuel will be transformed into ash, and as 1 kg of U235 is about equivalent to 3 000 000 kg of coal, then the mass of ash generated and dispersed to the environment is immense. Other forms of waste scales in a similar manner like the plastic and carbon fiber related to other forms of energy generation. But while high-level radioactive waste vanished with time, plastic and carbon fiber are not biodegradable or photo-degradable and will remain in the environment forever.
In summary, high-level radioactive waste contains fission products and minor actinides. Fissions products with shorter half-life time decays emitting gamma rays and are a major concern for the handling of high-level radioactive waste. However, after 300 years most of these isotopes will despair. The minor actinides with longer half-life time decays emitting alpha particles and they are dangerous if they are swallowed.
High-level radioactive waste will remain radioactive forever but after 500 years you can stand close to high-level radiative waste. A deep geological repository provides a way for storing high-level radioactive waste in a stable geologic environment.
Further reading
- What is Radiation: https://www.iaea.org/newscenter/news/what-is-radiation
- Radioactive Waste – The Journey to Disposal: https://www.iaea.org/newscenter/multimedia/videos/radioactive-waste-journey-disposal
- Immersive visit to Orano: https://www.orano.group/en/nuclear-expertise/immersive-visit
- What is nuclear waste, and what do we do with it? https://world-nuclear.org/nuclear-essentials/what-is-nuclear-waste-and-what-do-we-do-with-it
- Podcast by Deep Isolation- Nuclear Waste: The Whole Story: https://www.deepisolation.com/nuclear-waste-podcast/
- Dangers of Button Batteries and Kids: https://www.hopkinsmedicine.org/health/wellness-and-prevention/dangers-of-button-batteries-and-kids#:~:text=Why%20is%20swallowing%20button%20batteries,and%20dissolves%2Fburns%20the%20skin.
About

Carlos Alberto Dorao is Professor at NTNU – Department of Energy and Process Engineering. He has an engineering degree in Nuclear Engineering from Balseiro Institute, Argentina, and a PhD degree in Chemical Engineering from NTNU. He as amongst others worked as a researcher at the Bariloche Atomic Center, Argentina in the early 2000’s.


