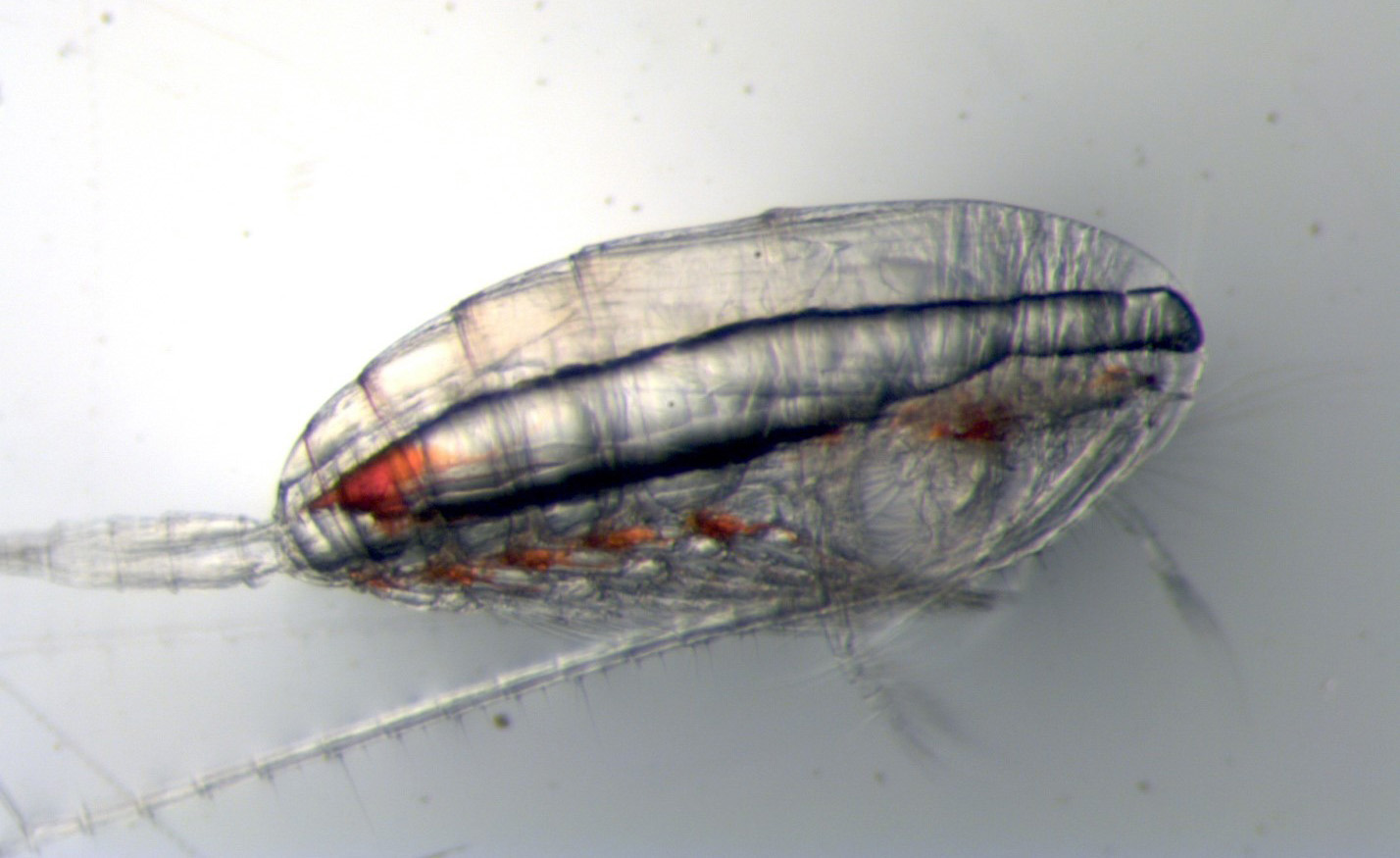
The marine ecosystem depends on zooplankton
The marine food web in the North Atlantic and Arctic Oceans depends on Calanus copepods, because they contain a lot of nutritious fat. A copepod is a type of marine zooplankton (“animal plankton”) drifting in the ocean. Copepods are distantly related to shrimp and crabs, and they belong to the group of animals called crustaceans. They accumulate a lot of fat (or lipids) in order to survive a period of inactivity or dormancy (called diapause), similar to a bear preparing for hibernation. They spend diapause at depth, slowly spending their lipid storage, and emerge in surface waters in time to feed on the spring phytoplankton (“plant plankton”) bloom1. Species like cod and herring time their reproduction cycles to the re-emergence of copepods, which are essential prey2.
Collecting copepods in diapause
Copepods spending the winter at 500 meters depth are not isolated from human-made disturbances. They may be exposed to toxic oil components from leakages from oil transportation pipes on the seabed, or from simply spending dormancy in the proximity of an oil platform that releases produced water at depth. In 2016, we performed an experiment where copepods in diapause from the Trondheimsfjord were exposed to toxic oil compounds (polycyclic aromatic hydrocarbons, PAHs) while they terminated diapause and developed into adults in laboratory conditions. Using RNA sequencing and bioinformatic software, we identified a large set of genes related to lipid metabolism in Calanus finmarchicus (norsk: raudåte) and evaluated their expression in response to oil exposure.


Lipid metabolism and diapause
The lipids that are so essential as energy source for fish and other higher trophic level species, are also important for the copepods that need to survive months without feeding. Individual lipid content may also have a role as an internal cue for diapause entry, duration and termination. Specifically, the copepods may be triggered to terminate diapause when a lower threshold of lipids is reached during diapause3,4. This results in the copepods “waking up” and migrate to surface waters to molt into reproducing adults.
Oil exposure disrupts lipid metabolism in Calanus copepods
We observed that the copepods that were exposed to oil components during diapause termination spent their lipids in the lipid sac at a slower rate that un-exposed copepods. This reduced lipid catabolic rate was also reflected by the gene expression results: several genes involved in lipid catabolism – the process of breaking down lipid molecules – were downregulated in the oil-exposed copepods. This resulted in the oil-exposed copepods having more leftover lipids at the end of the experiment than the non-exposed group. In nature, copepods being exposed to petrogenic oil may thus reach the lipid threshold for diapause termination at a later point in time. Exposure to oil pollution during this critical life phase can result in a delayed emergence from diapause, which can impact the entire marine ecosystem.
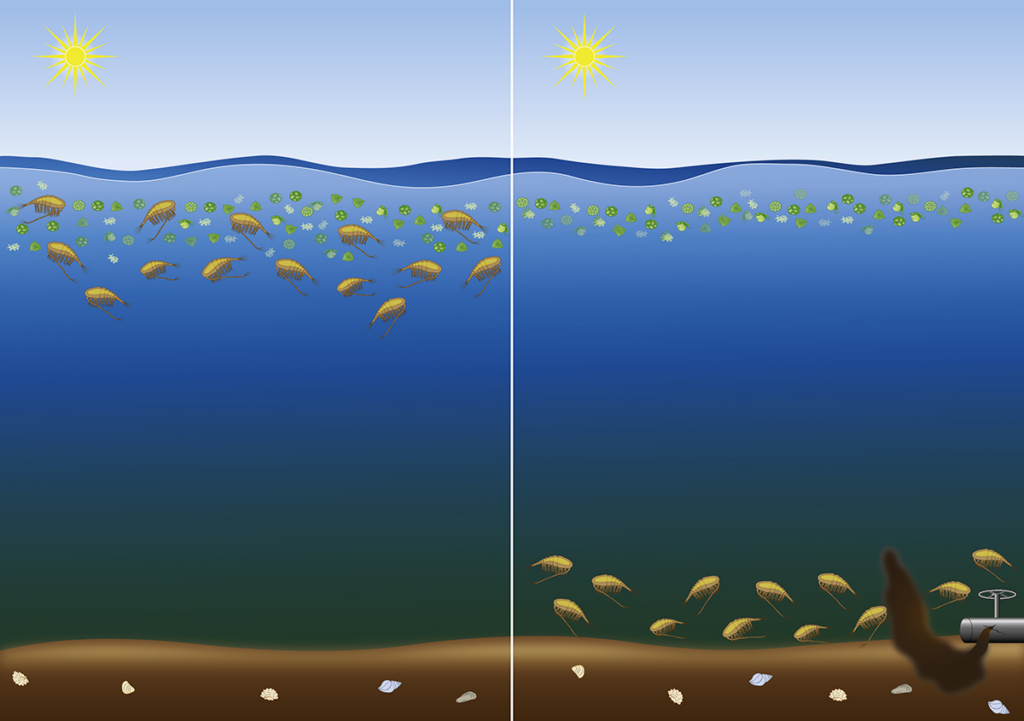
Severe potential ecological consequences
A delayed emergence to the surface can result in copepod populations missing the spring phytoplankton bloom, which is their primary source of food. Starvation during the reproductive period can limit the copepods’ ability to produce eggs5, and the survival of Calanus populations may be critically reduced if the timing is shifted drastically. Lower copepod availability in the surface waters at the time of hatching of cod larvae means less prey available and potentially lower survival of cod. In conclusion, ecosystem dynamics may be altered dramatically in a bottom-up effect in areas with chronic emissions of oil components.
These potential ecological consequences are of particular importance in high latitude areas with a short period of high biomass production. The Norwegian Government has opened for several new areas for oil exploration in the Barents and Norwegian Seas6. Further oil production will increase in the probability of accidental leakages and accidents, with increased risk to cause harm to an ecologically vulnerable Arctic area. Further studies are needed to reproduce our results, and to quantify the potential ecological consequences, in the Atlantic and the vulnerable Arctic marine ecosystems.
References
- Hirche, H.-J. Diapause in the Marine Copepod, Calanus Finmarchicus – A Review. Ophelia 1996, 44 (October), 129–143. https://doi.org/10.1080/00785326.1995.10429843.
- Beaugrand, G.; Brander, K. M.; Alistair Lindley, J.; Souissi, S.; Reid, P. C. Plankton Effect on Cod Recruitment in the North Sea. Nature 2003, 426 (6967), 661–664. https://doi.org/10.1038/nature02164.
- Johnson, C. L.; Leising, a W.; Runge, J. a; Head, E. J. H.; Pepin, P.; Plourde, S.; Durbin, E. G. Characteristics of Calanus Finmarchicus Dormancy Patterns in the Northwest Atlantic. ICES J. Mar. Sci. 2008, 65 (3), 339–350. https://doi.org/10.1093/icesjms/fsm171.
- Maps, F.; Plourde, S.; Zakardjian, B. Control of Dormancy by Lipid Metabolism in Calanus Finmarchicus: A Population Model Test. Mar. Ecol. Prog. Ser. 2010, 403, 165–180. https://doi.org/10.3354/meps08525.
- Hirche, H.-J.; Meyer, U.; Niehoff, B. Egg Production of Calanus Finmarchicus: Effect of Temperature, Food and Season. Mar. Biol. 1997, 127 (4), 609–620. https://doi.org/10.1007/s002270050051.
- Energidepartementet, O. Utlysning Av 25. Konsesjonsrunde. Pressemelding.
Further reading
- Skottene, Elise; Tarrant, Ann; Olsen, Anders Johny; Altin, Dag; Hansen, Bjørn Henrik; Choquet, Marvin; Olsen, Rolf Erik; Jenssen, Bjørn Munro. (2019) A Crude Awakening: Effects of Crude Oil on Lipid Metabolism in Calanoid Copepods Terminating Diapause. The Biological Bulletin. vol. 237 (2).
- Skottene, Elise; Tarrant, Ann M.; Olsen, Anders Johny; Altin, Dag; Østensen, Mari-Ann; Hansen, Bjørn Henrik; Choquet, Marvin; Jenssen, Bjørn Munro; Olsen, Rolf Erik. (2019) The β-oxidation pathway is downregulated during diapause termination in Calanus copepods. Scientific Reports. vol. 9 (1).